
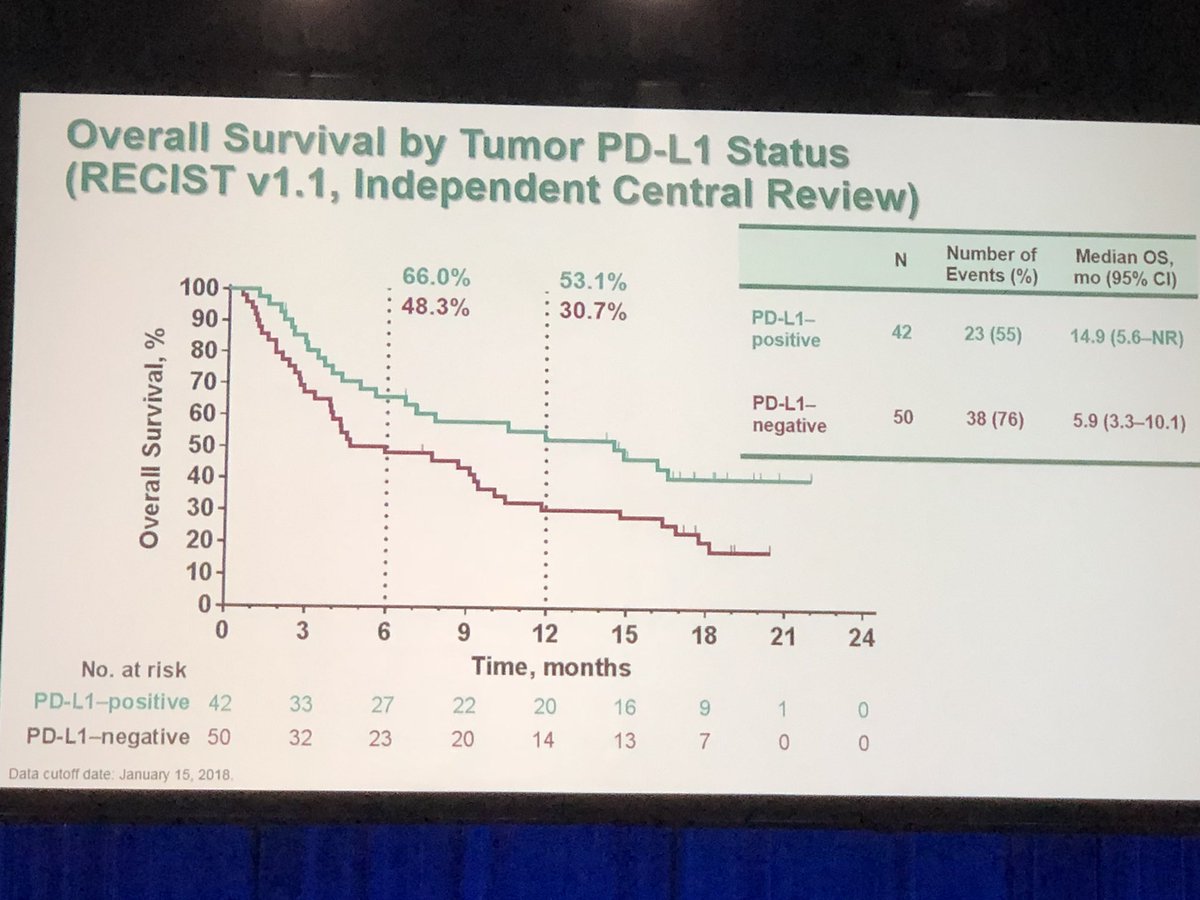
Of the 46 patients with cervical cancer screened, 39 were PD-L1+, and 24 were finally treated, all of them with metastatic cervical disease. Patients were treated with pembrolizumab 10mg/kg IV every 2 weeks for up to 2 years or until disease progression or intolerable toxicity. In this trial, patients must have failed standard-of-care systemic therapy, had a good performance status, and had a PD-L1-positive cervical cancer, defined as a PD-L1 expression ≥1% in tumor cells using immunohistochemistry. We discuss here the results of the KEYNOTE-158 study in this dedicated editorial commentary.įirst results published of pembrolizumab in advanced cervical cancers were data from the KEYNOTE-28 study designed to assess the efficacy and safety of pembrolizumab in 20 PD-L1+ advanced solid tumor cohorts ( 9). Based on the KEYNOTE-158 results, the US Food and Drug Administration (FDA) approved pembrolizumab in this particular unmet needs population of advanced PD-L1+ cervical cancer patients who had experienced progression following chemotherapy. It was further assessed in a phase II basket study, the KEYNOTE-158 study, investigating the efficacy and safety of pembrolizumab in several cancer types. Pembrolizumab is a highly selective antibody, blocking the PD-1 pathway, that was firstly evaluated in patients with recurrent or metastatic PD-L1+ cervical cancers in a multicohort phase I clinical trial, the KEYNOTE-028 ( 9). It has also been shown that PD-L1 is unregulated in high-risk HPV-associated lesions like cervical intraepithelial neoplasia ( 7, 8). There is a good rational to develop immunotherapy in viral induce-cancers like HPV induce cancers because of the existence of tumor-specific viral antigens ( 6). Immunotherapies and especially immune checkpoint inhibitors have recently made a breakthrough in oncology, mainly in advanced settings, with the development of anti-programmed cell death protein 1 (PD-1) or anti PD-1 ligand (PD-L1) antibodies, showing impressive results and long lasting responses in many different types of cancer, succeeding in restoring an anti-tumor immune response. Yet prognosis remains poor for advanced cervical cancer patients with the absence of standard of care in the second and later lines, in the context of resistance to platinum-based chemotherapy, and new therapeutic options are awaited. However, bevacizumab also increases toxicity, with higher rates of grade 3+ thromboembolism and fistulas. 13.3 months with chemotherapy alone, P=0.004), along with improved ORR (48% vs.
Keynote 158 plus#
More recently bevacizumab, an antiangiogenic antibody, has been evaluated in association with standard chemotherapy (cisplatin plus paclitaxel or topotecan plus paclitaxel) for first line advanced cervical cancer, showing a significant improvement of OS (17.0 vs. In this latter setting, first-line treatment consists of cisplatin-based chemotherapy, with several combination regimens tested, the combination with paclitaxel remaining the current standard of care, showing improvements in overall response rate (ORR) and progression-free survival (PFS) in comparison with cisplatin single agent, but no statistical benefit in terms of overall survival (OS) ( 4). The prognosis is dramatically poor for recurrent and/or metastatic (R/M) cervical cancers, with a 5-year survival of less than 5% ( 3). While the majority of early-stage cervical cancer patients are eligible for curative surgery, locally advanced cervical cancer patients will often recur despite multimodal chemoradiotherapy treatment with concomitant platinum-based chemotherapy ( 2). In poor countries, where there is limited access to these preventive strategies, cervical cancer remains though the second more frequent cancer in term of incidence and the third cause of cancer death in women ( 1).


With the establishment of screening programs and vaccinations against the human papillomavirus (HPV), the aim of these preventive strategies was to diminish the incidence of cervical cancer, which has significantly decreased over the past years in several Western countries ( 1). Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. Email: on: Chung HC, Ros W, Delord JP, et al. Head, Department of Drug Development and Innovation (D 3i), Institut Curie, 26, rue d’Ulm, 75005 Paris, France. Policy of Dealing with Allegations of Research MisconductĬorrespondence to: Prof.

Policy of Screening for Plagiarism Process.


 0 kommentar(er)
0 kommentar(er)
